Tribology is the discipline that studies the friction, the lubrication and the wear of surfaces in contact and in relative motion. In the ambit of this discipline, the noun “hydraulics” corresponds to the term “oleo-hydraulics” or “oleo-dynamics” of the current technical Italian literature and oil is the liquid commonly used. In this article, we are analysing the aspects connected with the risk of fire and its toxicity.

Claudia Di Sante
Oil, which can be both mineral and synthetic, is the liquid generally used for the lubrication of parts in motion and for the energy transmission. Its main characteristics are viscosity, which directly influences the friction met while flowing through tubes and equipment, the lubricating power and the protection against the corrosion of the various components. Besides, its capacity of removing heat in the points where energy losses are generated, allows using very small motors with regard to the delivered power, with good possibilities of repeating starts and stops with high frequency, without needing particular or oversized devices. Oil substantially performs two functions: lubricating and transmitting mechanical energy.
Lubricating oil
With the name of lubricating oils, we identify a class of liquid mixtures for the lubrication of mechanical organs. From the chemical point of view, they are composed by a base of hydrocarbon nature and by a whole of additives, which can be of mineral, semi-synthetic and synthetic origin. The lubricating oil is obtained through the petroleum refinement, with the addition of special additives that confer new properties to the product, improving its characteristics and extending its life. As well-known, it is used in the mechanical organs of machinery and in motors to reduce friction between parts in reciprocating motion, to protect components from wear, to favour the heat dispersion and to prevent the deposition of eventual non-soluble residues into the oil itself, keeping them in suspension and depositing them in a filter intended for retaining them. As above said, lubricating oils are generally composed by a hydrocarbon base and by a whole of additives. These additives compose from 10 to 30% of the overall volume of the lubricant. The additivation is subdivided into packages for the improvement of viscosity, of the viscosity index, of detergence, to inhibit the foam formation or oxidizing, and they are generally shared by all lubricants today on trade.
Therefore, we can distinguish two types of bases:
- mineral: they are the bases obtained from the petroleum refinement;
- hydrogenated or semi-synthetic mineral: they are obtained through hydrogenation from mineral bases or from natural gas;
- synthetic: they are obtained through chemical synthesis and not from petroleum;
- regenerated: they are the lubricants collected by disposed oil consortia and, after accurate procedures of new refinement and additivation of the product, they are reintroduced on the market.
Oil as power transmission
Oil, besides lubricating, is used to transfer energy and then to move cylinders, motors, wheels and reduction gears, interacting with all parts of a circuit. For this reason, different types of oils are available on the market and they are chosen according to the various characteristics: viscosity, lubricating capacity, resistance to ageing, hygroscopicity, high flash point and low noxiousness. This hydraulics sector is clearly of fundamental importance, due to the capability of managing notable powers by using small-size low-weight components in comparison with other alternative technologies.
In short, a hydraulic system must have:
- a generator group, where the transformation of mechanical energy into hydraulic energy occurs;
- a control group, where the fluid is conditioned making it take determinate values of pressure and flow rate and distributing it wherever necessary;
- a use group, formed by actuators of different kinds (cylinders, motors…).
Classification of hydraulic fluids
Now, after this fast survey of the applications of fluids in hydraulics, let us start considering the characteristics of hydraulic fluids. Although oil is considered the lubricating oil by antonomasia, for a complete treatment we should specify there are different hydraulic fluids, which can be used in different operational situations and can be grouped in four following fundamental groups:
- Water: normal industrial water is used or, when we want to attenuate its oxidizing power, in emulsion with 3-5% oil;
- Oils: they have a mineral base, i.e. they come from the petroleum refining and they are generally improved with the addition of special additives;
- Synthetic fluids with water base: they are emulsions of water in oil, composed by a high percentage of emulsifiable oil derived from petroleum, generally mixed in equal parts, containing special stabilizing anti-rust and anti-wear additives…; as alternative, there are water-glycol emulsions, generally composed by 40% of glycol and 60% of water, with the addition of additives;
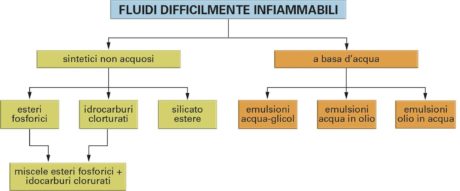
- Non-aqueous synthetic fluids: among the most common, it is worth reminding simple or chlorinated phosphoric esters, chlorinated hydrocarbons and ester silicates.
Characteristics of hydraulic fluids
The need of relying on hardly flammable fluids became pressing in the years around 1950, when it was evident that numerous disasters in industrial plants had been caused, or worsened, by the flammability of the oils used in hydraulic equipment. In case of failure or leak, if the fluid used is a mineral oil and if it (combustible), in presence of air (comburent), gets in contact with an adequate source of ignition (trigger), we run the risk that the fire propagates quickly to the surrounding areas. As the oil is generally pressurized in the circuit and then it leaks in spray form, its flammability becomes even higher. Hardly flammable fluids have been formulated both to withstand ignition and to limit the flame propagation; the prevalence of one or the other characteristic depends on the particular fluid nature. With the exception of the fluids of Group 1, i.e. those with aqueous base, which can be considered the only really safe ones still today, when there are serious fire risks, all other hydraulic liquids can burn when determinate conditions occur.
Actually, the most serious fire dangers are generated by the following situations:
- fluid jets, caused for instance by tube breakdowns, directed to surfaces with high temperature and notable thermal capacity: an almost instantaneous combustion can happen, equivalent to flames’;
- fluid fall on surfaces with such temperature and thermal capacity as to determine self-combustion phenomena in time.
Mineral oils are the least safe from this point of view. In fact, they already burn at low temperature, because they have quite low flame and self-combustion temperatures; moreover, if they catch fire, they go on burning, propagating the flame and then worsening the danger situation.
The fluids of Group 3, i.e. water-oil and water-glycol emulsions, have a very different behaviour compared to mineral oils: the evaporation of water, contained in high percentage, tends in fact to suffocate the flame and prevents its propagation. The real combustion will occur only after all water has evaporated.
In the fluids of Group 4, i.e. non-aqueous synthetic, the flammability resistance is connected with their chemical composition. Concretely, in comparison with the fluids of Group 3, they feature higher safety, both because the attitude of not propagating the flame is not linked with the water content and because the self-ignition temperature is higher. It is worth highlighting that hardly flammable fluid does not mean absolutely incombustible fluid but a fluid that takes some time to ignite.
Flammability tests
Numerous flammability tests of hardly flammable fluids have been proposed but, as different fluids can produce different or even contrasting results according to the adopted test, in the cases requiring very high safety it is indispensable to turn to specific tests to assess the fluid resistance to the expected service conditions. Flammability tests can be subdivided into lab tests and tests simulated under practical use conditions. Lab tests include:
- flash point of fluid: that is to say the temperature at which the fluid catches fire in contact with a free flame;
- flash point of vapours: that is the minimum temperature at which a sufficient quantity of fluid has evaporated, in order to form with the environment air, at atmospheric pressure, a combustible mixture that catches fire in contact with a free flame;
- self-ignition or self-combustion temperature, i.e. the minimum temperature at which the fluid spontaneously catches fire. The test is carried out by making a fixed oil volume (0.07 cc) drip into a glass ampoule that is heated until the liquid spontaneously catches fire.
The tests simulated under practical use conditions encompass numerous types proposed by specific users (aviation, mining industry, iron and steel industry) or by Unification and Standardization Bodies.

Toxicity
The prolonged contact with certain synthetic fluids and sometimes also with mineral oils can cause skin diseases and irritations. Neither water-glycol emulsions generally imply problems from this point of view nor water-oil emulsions are dangerous because the emulsifiers used do not usually attack the natural fats of the skin. The vapours of synthetic fluids, especially of chlorinated types, are very toxic and then operators must avoid breathing them. On the other hand, the presence of such vapours in dangerous concentration is infrequent, because the vapour tension in such fluids is exceptionally low (by the order of the fraction of mercury millimetre at the temperature of one hundred centigrade degrees). The danger situation can then occur if the fluid accidentally gets in touch with very hot surfaces or with flames: the fluid decomposes owing to the high temperature, developing a bog quantity of dense whitish smoke. Such smoke is extremely irritating for the respiratory system and also causes violent nauseas, however, it seems that, if breathed only for short periods, it does not produce intoxication phenomena.



